FROM GENOME TO PROTEOME
All of the information related to our bodies is contained in our DNA. Each gene comprises several elements: a transcription initiation site, a promotor region, a terminal so-called consensus sequence, exons and introns. The exons constitute the protein-coding regions. There are non-coding regions in the introns and intergenic sequences that regulate the genome’s expression. (1)
In order for the sequence coding for a protein to be read and expressed, the DNA double helix must be exposed. This occurs thanks to the acetylation of histones, which are responsible for the packing of DNA.
In addition, demethylation of the DNA and a recognition signal must occur in order for transcription to be initiated. RNA polymerase is activated, giving rise to messenger RNA. Through splicing, the introns are separated, and the exons are united and maintained thus in mature mRNA.
LThe mRNA base triplet (codon) binds to its complementary anticodon on the transfer RNA, then travels to the ribosome, where the ribosomal RNA reads the sequence and begins to translate it for protein synthesis. This is necessary to form a given structure or take effect in a bodily function. (2)

RNA INTERFERENCE
After splicing, most of the non-coding sequences are broken down. Those that are not degraded constitute RNA interference (ARNi), which regulate gene expression.
Among the various forms of non-coding RNA, miRNAs (interfering micro-RNAs) are particularly important. Their biosynthesis takes place as follows:
1 ° Transcription of the miRNA gene by RNA polymerase II: a simple RNA molecule of a thousand nucleotides is obtained, known as pri-miRNA or primary miRNA.
It contains a non-coding sequence and a complementary sequence, allowing it to bind later.
2 ° Splicing by the Drosha protein: a double-stranded 20-nucleotide RNA is form, which takes on a “hairpin” structure, the pre-miRNA or precursor miRNA.
3 ° The pre-miRNA is transported to the cellular cytoplasm where, through the action of the Dicer ribonuclease, the mature miRNA sequence is generated.
The miRNA is transported to the ribosome where it acts to modulate protein synthesis, through perfect coupling with the target mRNA.
There, it modulates expression of the coding gene through the RISC (RNA-induced silencing complex) molecular machinery through the breakdown of mRNA or blockade of its translation (rendering it illegible to ribosomes). Protein synthesis is thus impeded. (3)
miRNAs play a key post-transcriptional role. But they can also have a pre-transcriptional role, influencing histones or DNA methylation. They act as an epigenetic mechanism of gene regulation. (4)
This natural mechanism of miRNAs, RNA interference, occurs constantly in our cells. And it is of great importance whether a transcriptional unit is expressed or its expression is silenced.

HOMEOSTASIS AND CELLULAR SELF-REGULATION
A healthy balance of our body’s inner workings leads to its proper functioning. Epigenetic factors (environment, nutrition, stress, miRNAs, etc.) leave their mark on our DNA and modulate gene expression.
miRNAs regulate cellular homeostasis and participate in the differentiation, proliferation, apoptosis and metabolism of cells. They are also involved in the stress response, angiogenesis, oncogenesis and cardiovascular processes. But they can also act as tumour suppressors.
There are a great many miRNAs in humans (we currently know about over 3,000), which regulate more than 50% of our functional genes. The existence of miRNAs has also been identified in other living things: animals, plants, fungi, etc. Other types of RNA interference are also involved: siRNA (small interfering), piRNA (piwi-associated), etc.

MICRORNAs AS BIOMARKERS
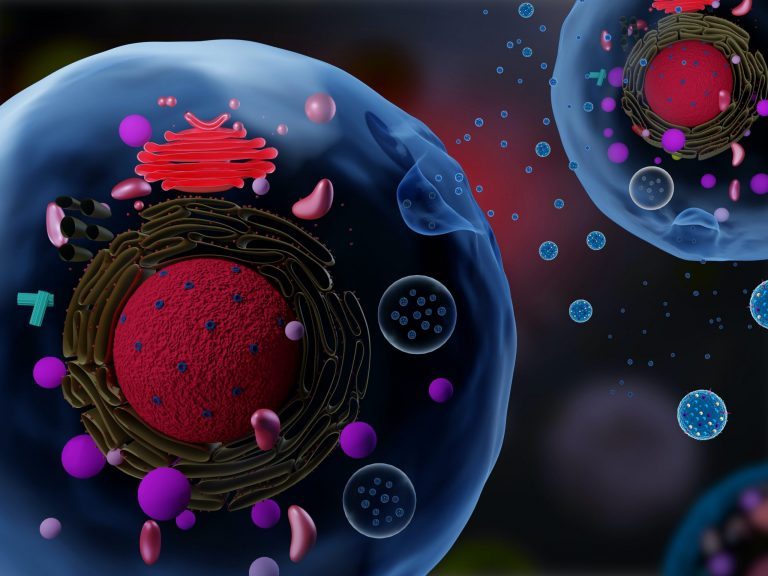
miRNAs can be detected in bodily tissues and fluids (serum, plasma, urine, saliva, etc.) and they are abundant in exosomes and microvesicles. (5)
They are associated with numerous diseases: Parkinson’s, osteoporosis, cancer, diabetes, hypertension, obesity, etc. As a result, they are useful as diagnostic biomarkers.
The dysregulation of miRNAs can lead to a failure of immune tolerance and subsequently to the development of an auto-immune disease. It can also influence the innate or adaptive immune response by activating or stopping an infection. The overexpression of miR-133a leads to the overexpression of RhoA in bronchial smooth muscle cells, thus promoting allergic asthma.
miRNAs can also act as prognostic markers or markers of advanced disease. For example, miR-181a and miR-21 are overexpressed in osteosarcoma. In the most aggressive form of this oncological disease, there is also overexpression of miR-221-3p and underexpression of miR-222-3p, which is not found in the forms with a better prognosis. In addition, MiR-199a is overexpressed in metastatic osteosarcoma.
There are several published databases regarding miRNAs: Plos One, miRBase, mirtarbase, starbase, microRNA.org, etc.

BIO IMMUNE(G)ENE MEDICINE AND CELLULAR SELF-REGULATION
We are at the gates of a future full of hope for the treatment of diseases, since we now know that miRNAs are involved in restoring cellular self-regulation and homeostasis.
The goal of Bio Immune(G)ene Medicine is not the introduction of a drug to treat an organ in one way or another, but to help cells re-establish the pathways and signalling processes that have become altered in the context of disease. In other words, to promote cellular auto-regulation.
By way of comparison, if a piece of music played by an orchestra does not appear to sound as it should, it is best to first of all establish whether the conductor of the orchestra has made an error in his interpretation of the piece or was distracted, before looking to repair or replace the instruments.
Bio Immune(G)ene Medicine uses nanovectors to introduce natural elements (since they exist in our cells) into the body, which are needed to restore the abnormal signalling pathways. In this way, we introduce miRNAs, as well as proteins, ligands, enzymes, hormones and antibodies. One single miRNA can regulate numerous target genes and thus influence multiple signalling pathways.
BI(G)MED uses nanomolecular doses, e.g. doses similar to those normally found in our cells. It is a question of bringing information to the sites of action, improving efficiency and avoiding side effects.
CONCLUSION
The discovery of RNA interference, and in particular miRNAs, constitutes a very significant step forward in the diagnosis and treatment of disease, due to their usefulness as biomarkers and therapeutic tools without the side effects that traditional pharmacological agents can cause.
BIBLIOGRAPHY
*https://en.wikipedia.org/wiki/MicroRNA
*https://www.exiqon.com/what-are-microRNAs
*Cavaillé, J. MicroRNA are everywhere. médecine/sciences. 2004. 20:p. 399-401
*Bernstein, E., Caudy, AA, et al. (2001) Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature 409 (6818): 363-6
*Bartel, DP. MicroRNAs: genomics, biogenesis, merchanism, and function. Cell. 2004 Jan 23; 116 (2): 281-97.
*Zeng, Y., Cullen, BR. (2005) Efficient processing of primary microRNA hairpins by Drosha requires flanking nonstructured RNA sequences. J.Biol Chem. 2005. doi:10.1074/jbc.M504714200
*Kim, VN. MicroRNA biogenesis: coordinated cropping and dicing. Nat Rev Mol Cell Biol. 2005 May; 6 (5): 376-85
*Luc, C, Tej, et al. Elucidation of the small RNA component of the transcriptome. Science. 2005 Sep 2; 309(5740): 1567-9
*Pillai, RS. MicroRNA function: multiple mechanisms for a tiny RNA? RNA. 2005 Dec; 11(12):1753-1761.
*Lewis, BP. et al. Conserveds seed pairing, often flanked by adenosines indicates that thousands of human genes are microRNA targets. Cell 2005 Jan 14; 120 (1):15-20
*Hayley, B. and Zamore, P.D. Kinetic analysis of the RNA: enzyme complex. Nat. Struct Mol. Biol, 2004. 11(7): p 599-606
*Ameres, S.L.,Martínez, J., and Schroeder, R. Molecular basis for target RNA recognition and cleavage by human RISC. Cell. 2007, 130 (1):p 101-12
*Rico-Rosillo, MG., et al. Importancia de los microARN en el diagnóstico y desarrollo de
enfermedades. Rev. Med Inst Mex Seguro Soc. 2014; 52(3):302-7
*González Martín, A. microRNAs: pequeños reguladores con un gran impacto en nuestro sistema inmune. http://dx.doi.org/10.18567/sebbmdiv_RPC.2019.10.1
*Sasaki, R. et al. Micro RNA-Based Diagnosis and Treatment of Metastatic Human Osteosarcoma. Cancers (Basel) 2019 Apr; 11(4):553
*Jones, K., Salah, Z. et al. MicroRNA signatures associate with pathogenesis and progression of osteosarcoma. Cancer Res. 2012 Apr 1; 72(7):1865-1877
*Calin, GA. Et al. Frequent deletions and down-regulation of micro-RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc Natl Acad sci USA. 2002 Nov 6;99(24):15524-9
FIGURES
1. Histone modification (Shutterstock)
2. Protein synthesis via transcription (Shutterstock)
3. miRNA biosynthesis (Wikimedia Commons)
4. Pre- and post-transcription regulation (Inmaculada Muñoz)
5. Exosomes detectable in fluids which may be useful for the diagnosis of disease. May contain miRNA, proteins, RNA, DNA and lipids of the original cell (Shutterstock)